Abstract
[Background]
A pre-emptive strategy has successfully decreased the incidence of cytomegalovirus (CMV) disease after allogeneic hematopoietic cell transplantation (HCT). However, it is difficult to completely prevent breakthrough CMV gastroenteritis, especially after acute graft-versus-host disease (GVHD) because a routine monitoring test with antigenemia or PCR assay often shows negative results before the development of CMV gastroenteritis that is considered as a localized infection initially. Actually, gastroenteritis is the predominant CMV disease in a pre-emptive strategy era. In addition, letermovir has recently been available for prophylactic strategies against CMV in clinical practice. However, little is known about the incidence, prognostic factors, and impact of subsequent CMV gastroenteritis after acute GVHD under recent advances in HCT including the introduction of letermovir.
[Methods]
This nationwide retrospective study evaluated adult patients who received their first allogeneic transplantation between 2008 and 2019 and developed grade II-IV acute GVHD (G24GVHD). Patients with a CMV-seronegative donor and recipient were excluded. Weekly monitoring using pp65 antigenemia assay was performed from the time of engraftment. A diagnosis of CMV gastroenteritis was made by gastrointestinal symptoms with histological proof of CMV on biopsy samples. The day when patients developed G24GVHD was considered as day 0 in all analyses. Nonrelapse mortality (NRM) by day 365 was set as the primary end-point. Cox proportional hazards regression models were used in all multivariate analyses. The impact of CMV reactivation and gastroenteritis as a time-dependent covariate were graphically plotted using a Simon-Makuch method. This study was approved by the data management committee of the Japan Society for Transplantation and Cellular Therapy (JSTCT) and by the Institutional Review Board of Jichi Medical University Saitama Medical Center.
[Results]
In total, 3759 patients with G24GVHD fulfilled eligibility and were included in this analysis. The median age at HCT was 50 years (range, 16 to 74). Of the 3759 patients with G24GVHD, 1120 (29.8%) developed grade III-IV acute GVHD. Letermovir prophylaxis was administered in 275 patients (7.3%), and the median start timing was 1 day after HCT (range, -8 to 36). The median duration of letermovir administration was 91 days (range, 2 to 332). The median observation period of survivors with letermovir prophylaxis was 320 days from the development of G24GVHD.
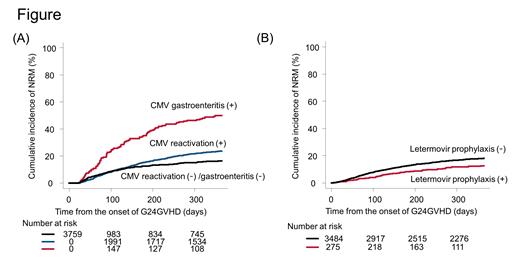
By day 365, 207 patients developed CMV gastroenteritis and the cumulative incidence was 5.7% (95% CI, 5.0-6.5%). The median duration between the development of G24GVHD and CMV gastroenteritis was 22 days (range, 1 to 235). Before the onset of CMV gastroenteritis, 37 (17.9%) did not develop CMV reactivation.
In multivariate analyses, advanced age (hazard ratio [HR], 1.60; 95% confidence interval [CI], 1.16-2.22; P = 0.004), GVHD prophylaxis using mycophenolate mofetil with calcineurin inhibitor (HR, 1.73; 95% CI, 1.08-2.77; P = 0.024), lower-gut acute GVHD at the development of G24GVHD (HR, 2.17; 95% CI, 1.58-2.98; P < 0.001), and use of systemic steroids (HR, 1.78; 95% CI, 1.16-2.74; P = 0.008) were independent risk factors for cytomegalovirus gastroenteritis. Moreover, CMV prophylaxis with letermovir was significantly associated with a decreased risk of CMV reactivation (HR, 0.25; 95% CI, 0.20-0.32; P < 0.001) and cytomegalovirus gastroenteritis (HR, 0.50; 95% CI, 0.25-0.99; P = 0.047). Then, we evaluated the impact of cytomegalovirus gastroenteritis on NRM by day 365. We found that patients who developed cytomegalovirus gastroenteritis (time-dependent covariate) had a higher risk of NRM (HR, 1.89; 95% CI, 1.50-2.39; P < 0.001) (Figure A). Meanwhile, letermovir prophylaxis reduced the risk of NRM (HR, 0.72; 95% CI, 0.52-0.99; P = 0.043). We illustrated the adjusted cumulative incidence of NRM in patients with and without letermovir prophylaxis in Figure B.
[Conclusion]
To our knowledge, this is the largest study summarizing the characteristics and outcomes of cytomegalovirus gastroenteritis after acute GVHD. Our findings underscore the importance of more stringent surveillance with endoscopy and prevention with letermovir based on a comprehensive risk assessment.
Kimura: SymBio Pharmaceutical: Honoraria; Takeda Pharmaceutical: Honoraria; Nippon Kayaku: Honoraria; Eisai: Honoraria; Ono Pharmaceutical: Honoraria; Bristol-Myers Squibb: Honoraria; Chugai Pharmaceutical: Honoraria; Kyowa Kirin: Honoraria; Pfizer: Honoraria; Astellas: Honoraria; Sumitomo Dainippon Pharma: Honoraria; MSD: Honoraria. Uchida: Chugai Pharmaceutical Co., Ltd.: Honoraria; Astellas Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd.: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Novartis Pharma Inc.: Honoraria. Nakamae: Astellas Pharma Inc.: Honoraria; Otsuka Pharmaceutical Co., Ltd: Honoraria; ONO PHARMACEUTICAL CO., LTD.: Honoraria; Simon-Kucher & Partners: Honoraria; Sumitomo Dainippon Pharma Co., Ltd.: Honoraria; Takeda Pharmaceutical Company Limited.: Honoraria; Novartis: Honoraria, Research Funding; Pfizer Japan Inc.: Honoraria; Bristol-Myers Squibb Company: Honoraria, Research Funding; Alexion: Research Funding; PPD-SNBL K.K: Research Funding; CMIC HOLDINGS Co., Ltd: Research Funding. Kanda: Sanofi: Research Funding; MSD: Honoraria; Otsuka Pharmaceutical: Honoraria, Research Funding. Atsuta: Mochida Pharmaceutical Co., Ltd.: Speakers Bureau; Astellas Pharma Inc.: Speakers Bureau; AbbVie GK: Speakers Bureau; Kyowa Kirin Co., Ltd: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria. Murata: GlaxoSmithKline: Honoraria; Asahi Kasei: Honoraria; Miyarisan Pharmaceutical: Honoraria; Astellas: Honoraria; JCR Pharmaceutical: Honoraria; Novartis: Honoraria; Toyama Chemical: Honoraria; FUJIFILM: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Kyowa Kirin: Honoraria; MSD: Honoraria; Celgene: Honoraria; Otsuka Pharmaceutical: Honoraria. Nakasone: Eisai: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Celgene: Honoraria; Bristol-Myers Squibb: Honoraria; Otsuka Pharmaceutical: Honoraria; Takeda Pharmaceutical: Honoraria; Chugai Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria.